Bilayer:
the double layer that makes the difference
An innovative membrane
Exaflex is a membrane obtained from the processing of the pericardium from Italian cattle aged less than 20 months, selected by breed and controlled in all stages of growth for food and environment in which they grow.
The unique qualities of Exaflex derive from the union between the raw material and our proprietary manufacturing process:
Fractional freeze-drying process: it helps to maintain the natural structure of the collagen fibers and facilitates optimal conditions for tissue integration, post-implantation.
Use of type I collagen: an essential element in the entire manufacturing process. It guarantees a low probability of causing immunological reactions.
Elimination of non-collagenous components, while preserving the active elements in the repair process: Proteoglycans, Hyaluronic Acid, Fibronectin, Elastin and native Collagen.
Thanks to these elements (a natural reservoir of bioactive factors), Exaflex controls inflammation, improves cell proliferation and migration.
Integration is also facilitated in the event of poor blood flow, because the quantity of implanted biological mass is limited (up to 50% less than the dermis matrices), maintaining high bio-mechanical performance.
- Exaflex is made with a proprietary process that maintains the particular natural properties of the pericardium.
- It provides rapid integration times with intrinsic cell growth, minimal inflammation and negligible serum production.
- It has a bilayer structure with a highly porous fibrillar layer capable of accommodating the cells. The matrix components of the layers work together with cytokines and growth factors for immediate revitalisation and early neoangiogenesis.
- The main purpose of the compact layer is to provide solid structural support. It is rapidly repopulated by fibroblasts and VEGF*. No accumulation of inflammatory or giant cells is noted after implantation.
* Vascular Endothelial Growth Factor (VEGF)
Types

Intact

Fenestrated
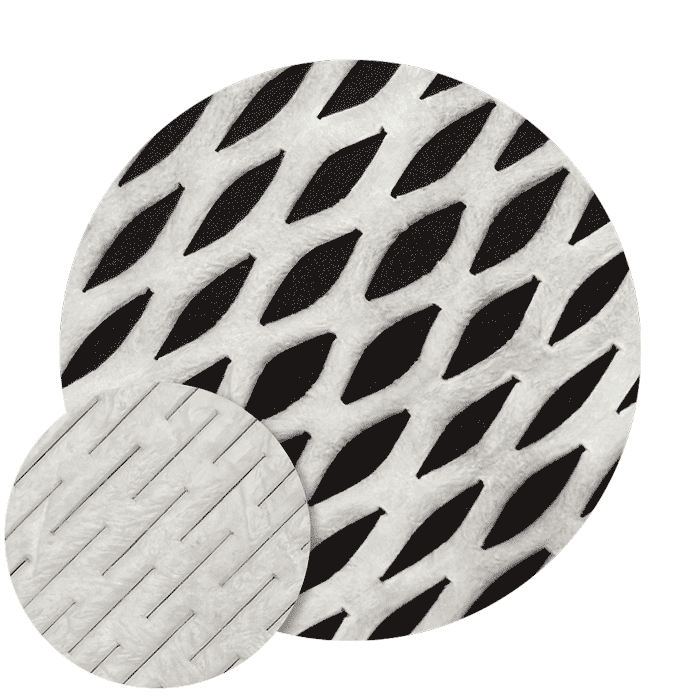
Mesh

Paste
Material safety
Materials of bovine origin are considered at BSE risk and, for this reason, the EC certification procedure is different from that of other types of animal grafts.
The certification procedure requires the processes to be approved and validated by all the Institutes of Public Health of the European Union Member States to allow the free movement of the material.
The checks consist of the following steps:
Analysis of the raw material origin and product traceability by a Notified Body.
Annual inspection of the sampling station by the Notified Body.
Check of the absence of cross-contamination during production.
Check and validation by the Institutes of Public Health of the prion charge deactivation and inactivation processes.
Quality system checks.
Authorisation from all European Ministries of Health to issue CE certification.
Surgical applications
Acellular matrix products can be used in a wide range of applications, including burns and reconstructive surgery, soft tissue and abdominal wall repair, and as internal implants for orthopedic use in joint surface reconstruction and joint repair tendons.